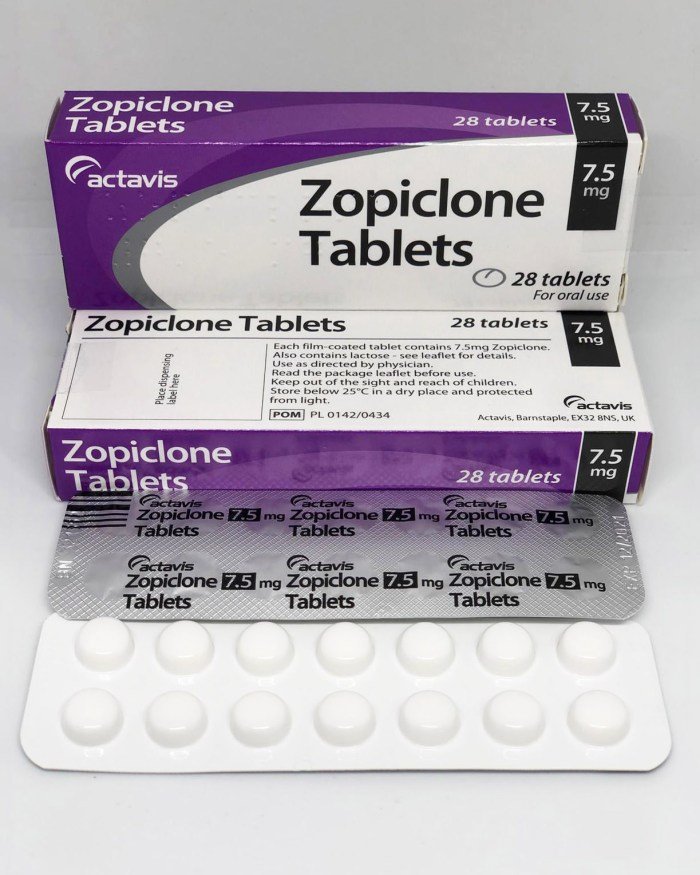
Zopiclone 7.5mg (UK Brand)
$65.00
Zopiclone 7.5mg is a prescription-only sleeping tablet, widely prescribed in the UK under brand names such as Zimovane®. It belongs to the ‘Z-drug’ class of sedative-hypnotics and is licensed for the short-term treatment (2-4 weeks maximum) of severe insomnia.
Zopiclone 7.5mg (UK Brand): A Detailed Profile of Britain’s Most Prescribed Hypnotic
Introduction
In the United Kingdom, Zopiclone 7.5mg (UK Brand)the 7.5mg tablet of zopiclone—most recognisably dispensed under the brand name Zimovane®—holds a unique and contentious position within the National Health Service (NHS) and the wider landscape of mental health prescribing. As the most commonly prescribed medication for insomnia in the UK, its distinctive pale blue tablet is a familiar sight in millions of British medicine cabinets. Yet, its prevalence belies a complex and often problematic reality. Zopiclone’s journey in the UK is a case study in the tensions between effective short-term symptom relief and the insidious public health challenge of iatrogenic dependence. This comprehensive 2000-word analysis will explore the specific context of zopiclone 7.5mg within the UK’s regulatory, clinical, and cultural framework, examining its pharmacology, its entrenched role in British general practice, the severe consequences of long-term use, and the ongoing efforts to curb its prescription in favour of safer, more sustainable alternatives.Zopiclone 7.5mg (UK Brand)
Chemical, Pharmacological, and Regulatory Profile in the UK
Class and UK Licensing Status
Zopiclone is classified pharmacologically as a cyclopyrrolone and is a benzodiazepine receptor agonist. In the UK, its legal status is crucial: it is a Prescription Only Medication (POM) and is further classified as a Schedule 4 Controlled Drug (CD) under the Misuse of Drugs Regulations 2001. This CD designation, shared with benzodiazepines, imposes strict recording and storage requirements on pharmacists and signifies the government’s recognition of its potential for misuse, dependence, and diversion. The standard NHS-licensed dose for adults under 65 is 7.5mg, which this analysis focuses on, with a 3.75mg dose licensed for the elderly and those with hepatic impairment.Zopiclone 7.5mg (UK Brand)
Mechanism of Action: The GABAergic Pathway
Zopiclone’s therapeutic action is mediated through the gamma-aminobutyric acid (GABA) system, the brain’s primary inhibitory network. It binds to the GABA-A receptor complex at a site adjacent to, but distinct from, the classical benzodiazepine binding site.
-
Positive Allosteric Modulation: Zopiclone functions as a positive allosteric modulator. It does not directly stimulate the receptor but enhances the effect of the brain’s own GABA. When zopiclone is bound, the GABA-A receptor undergoes a conformational change that makes it more responsive to GABA, leading to a greater influx of chloride ions into the neuron. This hyperpolarises the cell, making it less likely to fire, resulting in a net depressant effect on the central nervous system (CNS).
-
Receptor Selectivity (or Lack Thereof): Early marketing of “Z-drugs” like zopiclone emphasised their selectivity for receptor subtypes associated with sleep (α1 subunits). However, evidence now shows zopiclone binds with relatively low selectivity, also affecting α2 and α3 subunits linked to anxiety reduction and muscle relaxation. This gives it a broader pharmacological profile closer to traditional benzodiazepines, contributing to its anxiolytic effects and its wider range of side effects and risks.
Zopiclone 7.5mg
Pharmacokinetics: The “Intermediate-Acting” Profile
The pharmacokinetic properties of the 7.5mg tablet dictate its clinical use and its risks.Zopiclone 7.5mg (UK Brand)
-
Absorption and Onset: Zopiclone is rapidly absorbed after oral administration, with peak plasma concentrations occurring within 1-2 hours. This underlies its hypnotic effect, typically felt within 30-45 minutes, making it suitable for sleep-onset insomnia.Zopiclone 7.5mg (UK Brand)
-
Distribution and Metabolism: It is widely distributed and metabolised in the liver, primarily by the Cytochrome P450 enzyme CYP3A4. Its two main metabolites are N-desmethylzopiclone (weakly active) and zopiclone-N-oxide (inactive).
-
Elimination Half-Life: Approximately 5-6 hours. This “intermediate” half-life is its defining characteristic in the UK context:Zopiclone 7.5mg (UK Brand)
-
Therapeutic Implication: It provides coverage for both sleep initiation and maintenance, helping patients stay asleep for a significant portion of the night. This broad utility is a key reason for its popularity in primary care.
-
Adverse Effect Implication: The half-life is long enough to cause significant “hangover” effects the next day—drowsiness, dizziness, and impaired cognitive function. The most notorious and common side effect, reported by a substantial minority of UK patients, is a persistent, unpleasant metallic or bitter taste (dysgeusia), believed to be caused by excretion of the drug in saliva.
-
Dependence Implication: It is short enough to lead to interdose withdrawal anxiety in dependent users but long enough to allow some accumulation with nightly use, fuelling tolerance.
Zopiclone 7.5mg
-
Clinical Applications within the UK NHS
Licensed Indication and Prescribing Reality
The UK licence and the British National Formulary (BNF) are clear: zopiclone is indicated for the short-term treatment of insomnia, where it is severe, disabling, or subjecting the individual to extreme distress. The BNF explicitly recommends that treatment should be:Zopiclone 7.5mg (UK Brand)
-
Intermittent: If possible, used on only 2-3 nights per week.
-
Short-term: For a maximum duration of 4 weeks, including any dose-tapering period.
-
Initiated at the Lowest Effective Dose: 7.5mg for most adults, 3.75mg for those over 65.Zopiclone 7.5mg (UK Brand)
However, a chasm exists between guidance and reality. Data from NHS Digital and prescribing audits consistently show that hundreds of thousands of patients in England alone receive repeat prescriptions for zopiclone for months or years. This chronic use is not therapeutic; it is the management of prevented withdrawal, where rebound insomnia and anxiety are mistaken for the original condition.
The UK Primary Care Conundrum
The widespread long-term prescribing of zopiclone 7.5mg is a symptom of systemic pressures within the NHS:Zopiclone 7.5mg (UK Brand)
-
Time-Pressured Consultations: GPs, facing complex patients presenting with severe distress due to insomnia, may view a prescription as a swift, pragmatic solution compared to exploring underlying causes (stress, pain, mood disorders, lifestyle).
-
Limited Access to Alternatives: The first-line treatment for chronic insomnia, Cognitive Behavioural Therapy for Insomnia (CBT-I), suffers from notoriously long waiting lists within NHS Talking Therapies (IAPT) services. Faced with a distressed patient and a 6-month wait for therapy, the prescription pad is often the path of least resistance.Zopiclone 7.5mg (UK Brand)
-
The Dependence Cycle: A patient started on zopiclone for 2 weeks may find their original sleep problem returns with greater ferocity upon stopping (rebound insomnia). They report this to their GP, who perceives the drug as “working” and re-prescribes it, inadvertently initiating a long-term dependency.
Adverse Effects and Risks: The UK Experience
The side-effect profile of zopiclone is well-documented in UK pharmacovigilance and contributes significantly to its disease burden.
Common and Troublesome Effects:
-
Next-Day Sedation: Impaired alertness, coordination, and driving ability. The Driver and Vehicle Licensing Agency (DVLA) mandates that drivers must be warned of this effect, and it must be reported if alertness is compromised.
-
Dysgeusia: The metallic taste can be severe enough to lead to discontinuation.Zopiclone 7.5mg (UK Brand)
-
Cognitive Blunting: “Brain fog,” memory impairment (particularly anterograde amnesia), and reduced concentration.
-
Psychomotor Impairment: A major contributor to falls and fractures in the elderly, a significant concern for NHS falls services.Zopiclone 7.5mg (UK Brand)
Serious and Severe Risks:
-
Complex Sleep Behaviours: The Medicines and Healthcare products Regulatory Agency (MHRA) issues strong warnings. Patients may engage in sleepwalking, sleep-eating, or, most dangerously, sleep-driving, with no subsequent recollection. These events can occur with the first dose.Zopiclone 7.5mg (UK Brand)
-
Psychiatric and Paradoxical Reactions: Agitation, aggression, depression, hallucinations, and paradoxical increase in insomnia. These are more likely in the elderly and those with pre-existing psychiatric conditions.
-
Respiratory Depression: Can depress breathing, particularly hazardous for those with pre-existing conditions like COPD or sleep apnoea, and catastrophic if mixed with alcohol or opioids.
-
Tolerance, Dependence, and Withdrawal Syndrome: This constitutes the principal public health challenge.
Zopiclone 7.5mg
The Crisis of Dependence and Withdrawal in the UK
The UK is grappling with a silent epidemic of zopiclone dependence, estimated to affect a significant proportion of long-term users.
Mechanisms of Dependence:
-
Neuroadaptation: Chronic exposure leads the brain to downregulate GABA receptors and upregulate excitatory systems to compensate. The brain becomes dependent on the drug to maintain a new, artificial equilibrium.Zopiclone 7.5mg (UK Brand)
-
Tolerance: The 7.5mg dose loses its efficacy, leading patients to request dose increases (though 7.5mg is the maximum licensed single dose) or to take multiple tablets.
The Withdrawal Syndrome: Cessation unleashes a hyperexcitable nervous system. Symptoms, as detailed in UK clinical guidelines,Zopiclone 7.5mg (UK Brand) include:
-
Rebound Insomnia and Anxiety: Often more severe than the initial symptoms.
Zopiclone 7.5mg
-
Autonomic Hyperactivity: Sweating, tachycardia, tremor, nausea.
-
Perceptual Disturbances: Hypersensitivity to light/sound, tingling, feeling unreal.Zopiclone 7.5mg (UK Brand)
-
Severe Cases: Seizures, psychosis, delirium. Withdrawal can begin within 24-48 hours of the last dose due to its intermediate half-life.
The Difficulty of Discontinuation (“De-prescribing”): Stopping long-term zopiclone is notoriously difficult in primary care. Abrupt cessation is dangerous and unethical. A slow, individualised, patient-centred taper is required, often reducing by 3.75mg (half a tablet) every 1-2 weeks. This process can take months and is frequently marked by significant distress, requiring immense GP and patient commitment. Access to specialist support for medication withdrawal in the UK is extremely limited.
Zopiclone 7.5mg
UK Regulatory Context and Prescribing Initiatives
Recognising the scale of the problem, UK health authorities have launched concerted efforts to reduce inappropriate prescribing.
-
The BNF and NICE Guidance: Both are unequivocal about short-term use. NICE guidance on sleep disorders strongly advocates for CBT-I as first-line treatment.Zopiclone 7.5mg (UK Brand)
-
MHRA Warnings: Regular drug safety updates reinforce risks of dependence and complex behaviours.
-
NHS England’s “Stopping Over-Medication” Initiatives: Programmes like the NHS England & Improvement’s “STOPP” (Supporting Time to Produce Prescribing) and local Medicines Optimisation teams actively work with GP practices to audit hypnotic and anxiolytic prescribing, support tapering protocols, and promote non-drug therapies.
Zopiclone 7.5mg
-
Quality and Outcomes Framework (QOF): While not directly targeting zopiclone, QOF incentives for reviewing patients on long-term medications encourage GPs to scrutinise repeat prescriptions.Zopiclone 7.5mg (UK Brand)
The Path Forward: Alternatives and Safe Prescribing in the UK
Reducing harm from zopiclone 7.5mg requires a multi-faceted approach:
-
First-Line Treatment: CBT-I: Increasing access to CBT-I through IAPT and digital apps (like Sleepio, which is sometimes commissioned by NHS CCGs) is fundamental.Zopiclone 7.5mg (UK Brand)
-
Improved Patient and Clinician Education: GPs need training in “de-prescribing” and managing withdrawal. Patients need clear information at the point of prescription about the 4-week rule and risks of dependence—concepts enshrined in the NHS “Medicineswise” principles.
-
Structured Tapering Support: Developing clear, accessible local pathways and potentially dedicated community pharmacy support for supervised reduction.
Zopiclone 7.5mg
-
Addressing Root Causes: Ensuring insomnia is not treated in isolation, with proper assessment for and management of underlying depression, anxiety, pain, or lifestyle factors.Zopiclone 7.5mg (UK Brand)
Conclusion: A Pill Deeply Embedded, Slowly Being Uprooted
Zopiclone 7.5mg, in its familiar UK brand packaging, is more than a medicine; it is a symbol of a healthcare system’s struggle to manage a common,
Zopiclone 7.5mg
distressing condition within tight constraints of time and resources. Its intermediate half-life makes it an effective, convenient tool for the short-term management of severe insomnia, which explains its historical uptake.
However, its legacy is one of widespread iatrogenic harm. The tablet intended for a few nights’ respite has, for hundreds of thousands, become a prison of dependence, masking underlying issues and creating a withdrawal syndrome that is often worse than the original complaint. The bitter metallic taste it leaves is a fitting metaphor for the aftertaste of its long-term use.Zopiclone 7.5mg (UK Brand)
The future of zopiclone in the UK must be one of radically constrained, highly vigilant prescribing. It should be seen not as a first-line treatment, but as a limited, short-term crisis intervention,
Zopiclone 7.5mg
prescribed with the same caution as a controlled drug mandate, and always with a clear, discussed plan for review and discontinuation within weeks. The ongoing efforts of the NHS to shift focus from this potent chemical crutch towards the more challenging but sustainable foundation of CBT-I represent the only viable path out of the dependency trap. The story of Zimovane® in Britain is a powerful lesson in the law of unintended consequences in pharmacology and the enduring complexity of treating the mystery of sleep.Zopiclone 7.5mg (UK Brand)
| quantity | 28 pills |
|---|
Be the first to review “Zopiclone 7.5mg (UK Brand)” Cancel reply
Related products
sleeping pills
sleeping pills
sleeping pills
sleeping pills
sleeping pills
sleeping pills
sleeping pills
sleeping pills





Reviews
There are no reviews yet.