Blog
Alluvi Retatrutide
Alluvi Retatrutide: The Investigational Weight-Loss Drug Caught in a Web of Science, Scandal, and Illegal Sales
Meta Description: Explore the complete story of Alluvi Retatrutide—from its promising Phase 3 clinical trial results showing up to 28.7% weight loss to the massive illegal operation raided by UK regulators. What you need to know about this unapproved drug.Alluvi Retatrutide
Disclaimer: This article is for informational purposes only. Retatrutide is an investigational drug not approved for medical use by any regulatory agency. Alluvi-branded products are unlicensed and illegal. Always consult a licensed healthcare professional for weight-loss treatment options.Alluvi Retatrutide
Introduction
In the rapidly evolving world of obesity treatment, few compounds have generated as much excitement—or as much controversy—as retatrutide. Developed by pharmaceutical giant Eli Lilly, this investigational triple hormone receptor agonist has demonstrated remarkable results in clinical trials, with patients losing nearly 29% of their body weight in some studies . But alongside the legitimate scientific progress, a parallel and dangerous story has unfolded: the rise of illegal, unlicensed versions of the drug sold under the brand name “Alluvi.”Alluvi Retatrutide
This comprehensive guide explores both sides of the retatrutide story. We’ll examine the legitimate science behind this promising medication, including the latest Phase 3 trial results showing its potential for both weight loss and osteoarthritis pain relief. Then we’ll delve into the alarming investigation that uncovered what authorities described as the “world’s largest” illegal weight-loss drug operation, based in an unassuming industrial unit in Northampton, England .Alluvi Retatrutide
Understanding this complete picture is essential—not only for those excited about the future of obesity treatment but also for anyone tempted by the allure of purchasing these drugs online from unregulated sources. The gap between legitimate medical progress and dangerous counterfeit operations has never been wider, and the consequences of crossing that line can be severe.Alluvi Retatrutide
Part One: The Science of Retatrutide
What Is Retatrutide?
Retatrutide is an investigational drug developed by Eli Lilly and Company that belongs to a new class of medications known as triple hormone receptor agonists . Unlike earlier weight-loss drugs that target a single pathway, retatrutide simultaneously activates three different hormone receptors:Alluvi Retatrutide
- Glucose-dependent insulinotropic polypeptide (GIP) receptors
- Glucagon-like peptide-1 (GLP-1) receptors
- Glucagon receptors
This triple-action mechanism is what makes retatrutide potentially revolutionary. Each of these hormones plays a distinct role in metabolism:
GIP and GLP-1 are incretin hormones that stimulate insulin secretion, slow gastric emptying, and promote satiety. They’ve been the foundation of successful drugs like Mounjaro (tirzepatide) and Wegovy (semaglutide).Alluvi Retatrutide
Glucagon, by contrast, works differently—it increases energy expenditure by stimulating the breakdown of fat and glucose in the liver. By adding glucagon agonism to the mix, retatrutide may not only reduce appetite but also help the body burn more calories .Alluvi Retatrutide
The drug is administered as a once-weekly subcutaneous injection, similar to other incretin-based therapies. It’s currently being evaluated in several Phase 3 clinical trials under the TRIUMPH program, which has enrolled more than 5,800 participants across four global registrational trials .
The TRIUMPH-4 Trial Results: A Breakthrough in Obesity and Osteoarthritis
In December 2025, Eli Lilly announced positive topline results from the TRIUMPH-4 clinical trial, sending ripples through the medical community . This 68-week Phase 3 study evaluated retatrutide in adults with obesity or overweight who also suffered from knee osteoarthritis—a common and debilitating complication of excess weight.Alluvi Retatrutide
The results were striking:
Weight Loss Outcomes
| Endpoint | Retatrutide 9 mg | Retatrutide 12 mg | Placebo |
|---|---|---|---|
| Average weight loss | 26.4% (64.2 lbs) | 28.7% (71.2 lbs) | 2.1% (4.6 lbs) |
| Achieved ≥25% loss | 47.7% | 58.6% | 1.3% |
| Achieved ≥30% loss | 30.5% | 39.4% | 0.8% |
| Achieved ≥35% loss | 18.2% | 23.7% | 0.0% |
*Data source: Eli Lilly TRIUMPH-4 trial announcement *
From an average baseline weight of 112.7 kg (248.5 lbs) and BMI of 40.4 kg/m², participants receiving the 12 mg dose lost an average of 32.3 kg (71.2 lbs)—a level of weight loss previously unseen in clinical trials for obesity medications .
Beyond the Scale: Osteoarthritis Pain Relief
Perhaps equally impressive were the improvements in osteoarthritis symptoms. The trial measured pain using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), a validated patient-reported questionnaire where higher scores indicate worse pain.Alluvi Retatrutide
Pain Reduction Outcomes
- Retatrutide 9 mg: 4.5 point reduction (75.8% improvement)
- Retatrutide 12 mg: 4.4 point reduction (74.3% improvement)
- Placebo: 2.4 point reduction (40.3% improvement)
In a post-hoc analysis, approximately one in eight retatrutide-treated participants reported being completely free of knee pain at 68 weeks, compared to just over 4% of placebo recipients .
This matters because obesity and knee osteoarthritis form a vicious cycle: excess weight increases joint stress and inflammation, while pain limits mobility, making weight loss harder. By breaking this cycle, retatrutide could offer patients something no current medication provides: significant weight loss plus meaningful pain relief that might delay or prevent knee replacement surgery .Alluvi Retatrutide
Cardiovascular Benefits
The trial also measured markers of cardiovascular risk, with positive results:
- Reduced non-HDL cholesterol
- Lower triglycerides
- Decreased high-sensitivity C-reactive protein (a marker of inflammation)
- 14.0 mmHg reduction in systolic blood pressure at the 12 mg dose
These findings suggest that retatrutide’s benefits extend beyond weight and joint pain to potentially improve long-term cardiovascular outcomes—a critical consideration given that heart disease is the leading cause of death in people with obesity.
Safety Profile and Side Effects
No medication is without risks, and retatrutide’s side effect profile reflects its potent mechanism of action. The most common adverse events were gastrointestinal, consistent with other drugs in this class:
| Side Effect | Retatrutide 9 mg | Retatrutide 12 mg | Placebo |
|---|---|---|---|
| Nausea | 38.1% | 43.2% | 10.7% |
| Diarrhea | 34.7% | 33.1% | 13.4% |
| Constipation | 21.8% | 25.0% | 8.7% |
| Vomiting | 20.4% | 20.9% | 0.0% |
| Decreased appetite | 19.0% | 18.2% | 9.4% |
Of particular note was the occurrence of dysesthesia—altered sensation, often described as tingling or “pins and needles”—which occurred in 8.8% of patients on 9 mg and 20.9% on 12 mg, compared to just 0.7% on placebo . These events were generally mild and rarely led to treatment discontinuation, but they represent a side effect that differs from other incretin-based therapies.Alluvi Retatrutide
Discontinuation rates due to adverse events were 12.2% (9 mg) and 18.2% (12 mg), compared to 4.0% with placebo. Notably, these rates were highly correlated with baseline BMI—patients with higher BMIs were more likely to discontinue, including some who stopped because they felt they had lost too much weight .Alluvi Retatrutide
What’s Next for Retatrutide?
Retatrutide remains investigational and has not been approved for medical use by any regulatory agency, including the U.S. Food and Drug Administration (FDA) or the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) . This cannot be overstated: you cannot legally obtain genuine retatrutide from any pharmacy or prescriber.Alluvi Retatrutide
Eli Lilly is continuing its TRIUMPH clinical program, with seven additional Phase 3 trials expected to report results in 2026. These studies are evaluating retatrutide in:
- Obesity and overweight with weight-related complications
- Type 2 diabetes
- Knee osteoarthritis
- Moderate-to-severe obstructive sleep apnea
- Chronic low back pain
- Cardiovascular and renal outcomes
- Metabolic dysfunction-associated steatotic liver disease
If these trials prove successful, regulatory submissions could follow, potentially leading to approval sometime in 2027 or beyond. But for now, retatrutide exists only in the carefully controlled environment of clinical research.
Part Two: The Alluvi Story—Illegal Sales and Criminal Investigation
The October 2025 MHRA Raid
In late October 2025, enforcement officers from the MHRA arrived at an unassuming red-brick unit on a Northampton industrial estate . The location was unremarkable—wedged between an air-compressor service and an auto repair shop—but what they found inside was anything but.
Over a two-day raid, officers seized:
- Tens of thousands of empty injection pens, ready to be filled
- Raw chemical ingredients of unknown origin and purity
- Manufacturing equipment for assembling counterfeit medications
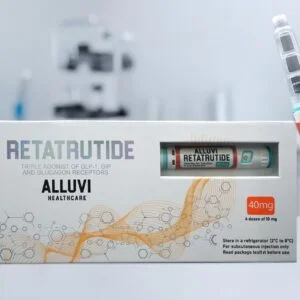
- Packaging materials branded with the “Alluvi” logo
- More than 2,000 unlicensed retatrutide and tirzepatide pens, already filled and packaged for sale
- £20,000 in cash
Authorities described the haul as the “world’s largest” of its kind involving illegal weight-loss drugs . Some of the seized pens were labelled as containing retatrutide—the powerful investigational drug still undergoing clinical trials, unapproved for medical use but widely hyped online as “the next Mounjaro” .
The Company Behind Alluvi
The company at the center of this operation is Alluvi Healthcare Limited. Despite the raid, the company’s website remained live months later, and over the Christmas period of 2025, it claimed products were unavailable due to “huge demand” rather than law enforcement action .Alluvi Retatrutide
The investigation by The Guardian revealed connections between Alluvi and a Northampton-based entrepreneur, Fasial Tariq, who has not been arrested or charged with any offense related to the operation . Records show the raided unit was registered to Wholesale Supplements Limited, of which Tariq is listed as director.
The trail of evidence includes:
- Ecommerce Nutri Collectiv—a website through which Alluvi products were sold, which later lost its payment provider after Stripe terminated its services
- Vantage Commercials Group Limited—a company once run by Tariq that shared a registered address with Nutri Collectiv
- Paradox Labs (formerly Paradox Studio)—a cryptocurrency venture founded by Tariq, to which the Nutri Collectiv website redirects
From Cryptocurrency to Counterfeit Drugs
Tariq’s background adds another layer of complexity to the story. His cryptocurrency project, Paradox Coin, and Paradox Metaverse—a “play-to-earn” blockchain game—attracted accusations of being a scam from online critics. The prominent crypto investigator Stephen Findeisen, known as Coffeezilla, publicly challenged Tariq in a YouTube interview, questioning the project’s economics and promotional claims .
Local residents in Northampton noted that luxury cars—including a Rolls-Royce and a distinctive bright-green Lamborghini Huracán Spyder—were frequently parked outside the raided unit . Tariq’s former company, Onyx, specialized in high-end car rental and chauffeur services.
Tariq also has a history of driving offenses. In 2018, a Ferrari traveling at more than 135 mph on the M6 motorway was overtaken by a BMW registered to his company Onyx Executive Travel Ltd. When Tariq failed to identify the driver, he was fined and later banned from driving for 12 months—having regained his license only days earlier after convictions for drink-driving and driving while disqualified .
The Ongoing Operation: No Arrests, Continued Sales
Perhaps most alarming is that months after the raid, no arrests have been made . While the industrial estate site is now closed, rumors suggest production may have simply shifted elsewhere.
Alluvi’s Telegram channel remains active, attracting thousands of members who appear to place orders daily. The company continues to offer products like “retatrutide 40mg x2 Bundle (R&D Only)” for £339.99 —despite the fact that legitimate retatrutide does not exist in any commercial form.
The “R&D Only” disclaimer is a common tactic used by illegal sellers to evade regulations, claiming products are for “research purposes” and “not for human consumption.” However, regulators explicitly state they disregard such claims if promotional material makes it clear the products are intended for human use . The Telegram channels and social media promotions selling these products to ordinary consumers leave no doubt about their intended purpose.
The Supply Chain: A Global Web of Illicit Manufacturing
An anonymous source with inside knowledge of the illegal weight-loss drug trade described the sector to The Guardian as chaotic, secretive, and poorly regulated . Those behind Alluvi were characterized as “nasty,” deliberately drawing attention to an underground supply chain that typically prefers to operate in the shadows.
The source revealed disturbing details about the operation’s supply chain:
- Boxes for Alluvi products were printed by a company in China
- Orders could be placed easily through various online channels
- Products were assembled cheaply, with little concern for sterility or dosing accuracy
- Injection pens could be purchased readily from e-commerce websites and later filled with illicit substances
This globalized, fragmented supply chain makes enforcement extraordinarily difficult. Products cross borders as raw materials, components, and finished goods, exploiting gaps in regulatory oversight and legal grey areas.Alluvi Retatrutide
The Scale of the Problem: A Booming Black Market
The Alluvi case is not an isolated incident—it represents a growing crisis. The black market for weight-loss medications is booming, driven by a perfect storm of factors:Alluvi Retatrutide
- High demand from millions of people seeking effective obesity treatment
- Limited access on public health systems like the NHS
- Prescription requirements that create barriers to access
- Hefty price tags for legitimate medications
- Social media promotion that reaches vulnerable consumers directly
In October 2025, the MHRA revealed it had seized more than £250,000 worth of counterfeit weight-loss jab products from the Northampton factory alone . Banks have also warned of scams relating to weight-loss medications, noting that victims lose £120 on average.
The MHRA has issued repeated warnings about beauty salons, fake pharmacy websites, and social media posts selling prescription weight-loss medications without a prescription—an illegal practice. Key signs to watch out for include:Alluvi Retatrutide
- Social media posts offering unusually low prices
- Promises of “miracle results” or “quick fixes”
- Sellers who do not require a prescription
- Products sold through Telegram channels or other encrypted messaging apps
Part Three: The Critical Gap—Science vs. Crime
Why You Cannot Buy Real Retatrutide
It’s essential to understand: there is no such thing as commercially available retatrutide. The drug exists only in two places:Alluvi Retatrutide
- Controlled clinical trials run by Eli Lilly, with strict oversight, ethical approval, and medical supervision
- Illegal manufacturing operations like Alluvi, producing counterfeit versions of unknown origin, purity, and safety
Anyone selling retatrutide online is, by definition, breaking the law and endangering lives. The “Alluvi Retatrutide” seized by the MHRA bears no relationship to the genuine investigational drug being studied by Eli Lilly—it is an counterfeit product, made in unsterile conditions with raw ingredients of unknown quality.
The Dangers of Unlicensed Weight-Loss Jabs
Medical experts warn that the risks of using unregulated injectable drugs are severe and potentially life-threatening :
Contamination and Infection
Products manufactured in unregulated facilities are not sterile. They may contain bacteria, fungi, or other pathogens that can cause severe infections at injection sites or systemic infections requiring hospitalization .
Incorrect Dosing
The pens may contain the wrong amount of active ingredient—either too little (rendering them ineffective) or too much (causing dangerous blood-sugar fluctuations, pancreatitis, or overdose). The anonymous source familiar with Alluvi’s operations alleged that products were assembled with “little concern for sterility or dosing accuracy” .
Unknown Ingredients
Counterfeit drugs may contain different ingredients than advertised—or no active ingredient at all. They could include dangerous additives, fillers, or completely different drugs that interact unpredictably with other medications.Alluvi Retatrutide
Temperature Instability
These drugs are made from living organisms and are highly sensitive to temperature variations. Jason Murphy, a weight-loss expert and head of pharmacy at Chemist4U, warned of potential complications during winter: “These drugs are made from living organisms and are highly sensitive to temperature variations and extreme highs or lows” . Improper storage during shipping—especially in cold weather—can destroy efficacy or make the drugs dangerous.
No Medical Oversight
Legitimate weight-loss medications require proper medical supervision—baseline health assessments, monitoring for side effects, dose titration, and ongoing support. Buying from illegal sellers bypasses all of this, leaving patients to manage complications alone.Alluvi Retatrutide
Regulatory Warnings and Government Action
The MHRA has been unequivocal in its warnings. Jenn Matthissen, of the MHRA’s safety and surveillance team, stated: “People often look for ways to support their health at this time of year but buying medicines from illegal online sellers can put your health at real risk. Always make sure you are using authorised products from legitimate sources and speak to a healthcare professional for advice on safe, evidence-based options” .
The UK’s health minister, Dr. Zubir Ahmed, added a stark warning: “As a practising doctor and patient safety minister, I want to be absolutely clear: please do not buy weight loss medications from unregulated sources. These products are made with no regard for safety or quality and pose a major risk to unwitting customers. Don’t line the pockets of criminals who don’t care about your health. Safe, appropriate, licensed obesity drugs can greatly benefit those with a clinical need but should be obtained from a registered pharmacy against a valid prescription” .
The “Research Chemical” Loophole Is a Lie
Sellers like Alluvi often claim their products are for “research purposes only” or “not for human consumption” to evade regulations. The MHRA explicitly states they disregard such claims if promotional material makes it clear the products are intended for human use . When a company advertises weight-loss pens on Telegram channels frequented by consumers seeking obesity treatment, there is no ambiguity about intent.
Dr. Emily Rickard, a research fellow at the University of Bath, highlighted the enforcement gap: “In early September, I reported what appeared to be an illegal website selling retatrutide to the British public after seeing a Facebook advert. Facebook removed the advert quickly, but more than two months later the website is still live. That sets a deeply concerning precedent for patient safety” .
Her colleague, Dr. Piotr Ozieranski, added: “At present, it often feels that the worst that can happen is a slap on the wrist. Meanwhile, the public remains exposed to serious harm. Regulators should proactively investigate suspected unethical practices and impose fines linked to company turnover. Right now, the system feels as though it protects suppliers more than patients” .
Part Four: What Patients Need to Know
Legitimate Options for Weight-Loss Treatment
If you’re struggling with obesity and considering medical treatment, there are safe, legal, and effective options available today:Alluvi Retatrutide
Approved GLP-1 Medications
Several medications are already approved by regulators worldwide for weight management:
- Wegovy (semaglutide) —approved for chronic weight management
- Mounjaro (tirzepatide) —approved for type 2 diabetes and increasingly used for weight loss
- Saxenda (liraglutide) —an earlier GLP-1 option
- Orlistat —a non-injectable option that works differently
These medications require a prescription and medical supervision, but they are manufactured under strict quality controls, stored properly, and dispensed by licensed pharmacies.
Clinical Trials
For those interested in investigational drugs like retatrutide, participating in a clinical trial is the only safe and legal way to access them. Clinical trials:Alluvi Retatrutide
- Are conducted under strict ethical oversight
- Provide free medical monitoring and care
- Ensure participants understand the risks and benefits
- Contribute to scientific knowledge that helps future patients
Information about ongoing clinical trials can be found at ClinicalTrials.gov or through major academic medical centers.
Comprehensive Treatment Programs
Medication works best as part of a comprehensive approach that includes:
- Dietary counseling
- Physical activity guidance
- Behavioral support
- Management of obesity-related complications
Red Flags: How to Spot Illegal Sellers
Protect yourself by watching for these warning signs:
🚩 No Prescription Required
Legitimate weight-loss medications always require a prescription from a licensed healthcare provider based on a proper medical evaluation.
🚩 Sold on Social Media or Telegram
Legal medications are dispensed by registered pharmacies—not through Instagram ads, Facebook groups, or encrypted messaging apps.
🚩 “Research Only” Disclaimers
If a seller claims a product is “for research purposes” but markets it for weight loss, they’re trying to exploit a loophole that regulators explicitly reject.
🚩 Prices That Seem Too Good to Be True
Legitimate medications are expensive to develop and manufacture. Unusually low prices almost always indicate counterfeit products.Alluvi Retatrutide
🚩 Lack of Physical Pharmacy Address
Registered pharmacies have physical premises you can verify. Be suspicious of sellers who only have a website or Telegram presence.
🚩 Pressure to Buy Quickly
Scammers often create false urgency—”limited supply,” “huge demand,” “last chance”—to pressure you into making a hasty decision.
🚩 Vague or Missing Product Information
Legitimate medications come with detailed patient information leaflets, clear dosing instructions, and verified expiration dates.
What to Do If You’ve Purchased From an Illegal Source
If you’ve already bought weight-loss jabs from an unregulated source:
- Do not use the product. The risks of contamination, incorrect dosing, and unknown ingredients are simply too high.Alluvi Retatrutide
- Consult a healthcare professional. Explain what you’ve purchased and ask for advice. They can assess your health status and watch for potential complications.
- Report the seller. In the UK, you can report illegal medicine sellers to the MHRA. In the US, report to the FDA. Your report could help prevent others from being harmed.
- Monitor your health. If you’ve already used the product and experience symptoms like severe nausea, abdominal pain, signs of infection at injection sites, or unusual reactions, seek medical attention immediately.
Part Five: The Future of Retatrutide
What Legitimate Approval Might Look Like
Assuming ongoing trials continue to show positive results, retatrutide could eventually become available as a prescription medication. The timeline remains uncertain, but experts suggest:
- 2026: Additional Phase 3 trial results expected across multiple indications
- 2027-2028: Potential regulatory submissions and reviews by FDA, MHRA, and other agencies
- 2028+: Possible commercial launch if approved
When and if approved, retatrutide would join the growing class of incretin-based therapies for obesity—but with the distinction of being the first triple agonist to reach market.
The Potential Impact on Obesity Treatment
If retatrutide’s Phase 3 results hold up in further studies and translate to real-world practice, the implications are profound:
- Near-surgical levels of weight loss without the risks of bariatric surgery
- Dual benefit for osteoarthritis that could reduce the need for joint replacement
- Cardiovascular improvements that address the leading cause of death in obesity
- A new treatment paradigm for obesity as a chronic disease requiring long-term management
Dr. Kenneth Custer of Eli Lilly summarized the company’s optimism: “We are encouraged by the results of TRIUMPH-4, which highlight the powerful effect of retatrutide, a first-in-class triple agonist, on body weight, pain and physical function. With seven additional Phase 3 readouts expected in 2026, we believe retatrutide could become an important option for patients with significant weight loss needs and certain complications, including knee osteoarthritis” .Alluvi Retatrutide
The Challenge of Counterfeits Will Only Grow
As legitimate obesity medications become more popular and more effective, the counterfeit market will likely expand in parallel. The Alluvi case may be the largest such operation uncovered to date, but it won’t be the last.
Regulators face significant challenges:
- Global supply chains that cross multiple jurisdictions
- Encrypted communication channels that are difficult to monitor
- Payment systems that can be quickly reestablished after shutdowns
- Social media platforms where illegal sellers can reach millions
Dr. Ozieranski’s call for proactive enforcement rather than reactive complaints reflects the scale of the challenge. By the time a complaint is filed and investigated, illegal sellers may have already moved on—taking new identities, new websites, and new customers with them .
Conclusion: Two Paths, One Destination
The story of Alluvi Retatrutide is really two stories intertwined.
One is a story of legitimate scientific progress—of researchers at Eli Lilly developing a novel molecule that could transform the treatment of obesity and its complications. The Phase 3 TRIUMPH-4 results showing 28.7% weight loss and dramatic improvements in osteoarthritis pain represent genuine medical advancement . For millions of people struggling with obesity, the prospect of an effective, well-tolerated medication is genuinely hopeful.Alluvi Retatrutide
The other story is darker—a tale of criminal exploitation, counterfeit manufacturing, and profound disregard for human safety. The individuals behind Alluvi allegedly set up an illegal factory in an industrial unit, assembled products with no concern for sterility or accurate dosing, and sold them through encrypted channels to vulnerable consumers seeking help with weight loss . They allegedly drove luxury cars funded by this trade while exposing customers to risks of infection, incorrect dosing, and unknown health consequences.
These two stories share a molecule—retatrutide—but diverge in every other respect. The genuine drug exists only in carefully controlled clinical trials. The counterfeit versions exist only in illegal supply chains that regulators are struggling to shut down.
The Takeaway
If you’re struggling with obesity and considering medical treatment:
Do not buy Alluvi Retatrutide or any unlicensed weight-loss product online. It is illegal, unapproved, and dangerous. The product seized by the MHRA bears no relationship to the genuine investigational drug—it is a counterfeit of unknown origin and unknown risk.
Consult a licensed healthcare provider. Discuss approved, regulated options that are manufactured under sterile conditions and dispensed by legitimate pharmacies with valid prescriptions. These medications work—they may not produce 28.7% weight loss (yet), but they are safe, effective, and properly monitored.
Be patient for legitimate options. If retatrutide proves successful in ongoing trials and gains regulatory approval, it will become available through proper medical channels. The wait is frustrating, but it’s infinitely preferable to risking your health on counterfeit products from criminal enterprises.
The gap between legitimate science and illegal exploitation has never been wider. Choose the path of safety, evidence, and proper medical care—your health depends on it.